The Pumpless Ion Exchange System

PIErs are a team of bright undergraduate water-enthusiasts from the Jomo Kenyatta University of Agriculture and Technology who seek to provide clean and safe drinking water for all. The following short video is a story of how we intend to provide a sustainable solution for the removal of industrial pollutants in water, using PIES, an ion-exchange system.
Background
Clean and sufficient drinking water is important for the survival of every human being. The World Health Organization recommends guidelines on the daily water requirement per person and the standard thereof. This is however theoretical, as various countries around the World face drinking water issues, both in terms of quantity and quality. The City of Ulsan, a major industrial hub in South Korea is a case in point, facing both a water quantity, and quality issue. This problem is attributed to political reasons around preservation of the Bangudae Petroglyphs, a cultural heritage site, and to an extent, competition of water from the heavy industries. Industrialization is a contributing factor, but cannot be considered as a problem, since Ulsan was designated as an industrial area thereby leading to the rapid growth of the city. This has in turn led to an increase in population over the years, thus increasing the water demand. The main reason for the current drinking water issue in Ulsan is therefore the preservation of the Bangudae Petroglyphs, which the government aims to register as a UNESCO World Heritage Site. In response to this, the Sayeon and Daegok dam water levels had to be lowered in order to prevent flooding of these ancient architecture, as this led to their deterioration. Sayeon dam, for instance, used to operate at an elevation level of 60 m but now operates at 52 m, below the petrolypghs level. This has in turn led to a water shortage issue over the years and the people of Ulsan were forced to draw water from the Nakdonngang River. The river passes through an industrial watershed and is therefore heavily polluted, thus leading to the secondary issue of low drinking water quality. The residents of Ulsan find this situation unfair as the petroglyphs were discovered in 1971, 6 years after the commissioning of the Sayeon Dam (1965). Numerous efforts have been put over the years in an attempt to solve this issue, such as protection of the petroglyphs and purification of the heavily polluted Nakdong River water. A sustainable solution is yet to be met, and this is what we seek to solve.
Ulsan City Drinking Water Challenge
- Insufficient drinking water supply, currently standing at about 270 litres per capita per day (l/c/d) compared to a demand of 340 l/c/d.
- Lack of clean drinking water forcing residents to purchase bottled water for drinking and cooking purposes.
- High cost of water as the local government of Ulsan pays the Korea Water Resources Corporation to draw water from Nakdonngang River. According to statistics from the local government, Ulsan pays approximately 30 billion KRW per year to use water from the river.
Our Direction
With most of the alternative water sources proposals posing challenges in terms of feasibility, viability and sustainability, we chose to focus on the intensive purification of Nakdonngang River water to make it safe for human consumption. According to our research, there are ongoing projects to offset the 120,000m3/day water shortage in Ulsan City. These include: additional water supply from Unmun Dam (70,000 m3/day) and conversion of Daeam Dam for drinking water supply (50,000 m3/day). It is therefore safe to say that by the year 2025 Ulsan City will not have a drinking water shortage. We therefore strongly believe that intensive water purification is the right step.
Our Solution
The current water treatment process employed on Nakdong River water is as shown in the figure below:
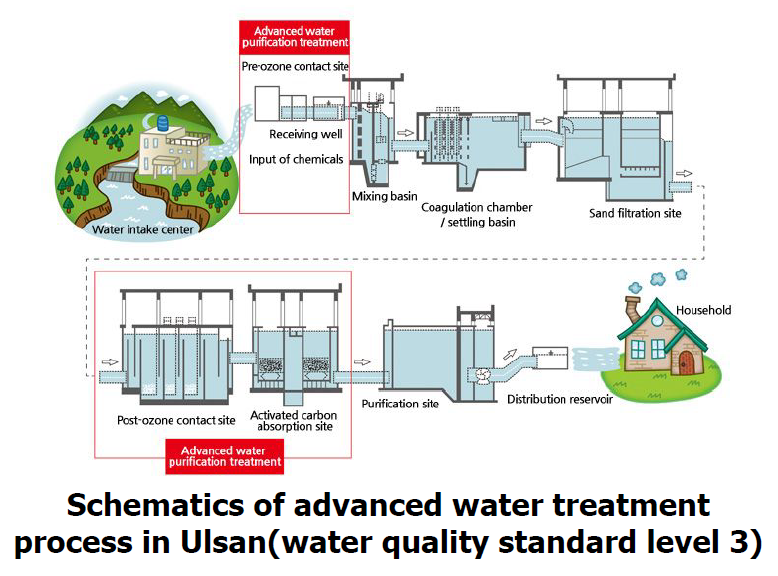
According to the schematics, the advanced water treatment processes currently in use are ozonation and activated carbon adsorption.
Ozonation (advanced oxidation) is a water treatment process that involves the use of ozone gas to remove industrial pollutants such as heavy metals and organic pollutants. These pollutants are oxidized leading to the formation of insoluble metal oxides and hydroxides, and increasing the biodegradability of organic carbons respectively. The former is subsequently removed using solid/liquid separation processes such as coagulation and sedimentation/filtration, whereas organic carbons are oxidized into carbon dioxide.
Activated carbon adsorption uses thermalized carbonaceous materials to remove industrial pollutants such as organic pollutants and heavy metals through adsorption.
These processes are however limited to the removal of insoluble compounds of a given particle size.
We therefore propose the use of the ion-exchange process which will remove both microscopic and dissolved industrial pollutants from the water thus purifying it.
Ion-exchange Water Treatment
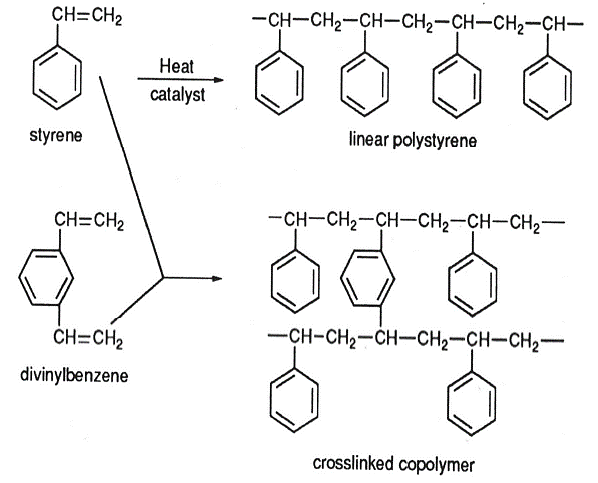
Ion-exchange is a process that involves the exchange of ions of the same charge between an insoluble compound (resin) and a solution. The insoluble compound is made up of natural or synthetic resins which contain cations and anions used in the exchange process, thus cationic and anionic exchange resins. These resins can be of a strong or weak form depending on the acid and base used in the formation of the functional groups. The polymer backbone of the resins is made up of either polystyrenic or polyacrylic compounds depending on the plant design and operating conditions. For special removal purposes which require a high selectivity, chelating resins are used.
Our system will utilize polystyrenic exchange resins based on sulphonic and quaternary amine functional groups for cation and anion removal respectively.
Sulphonic exchange resin formation:
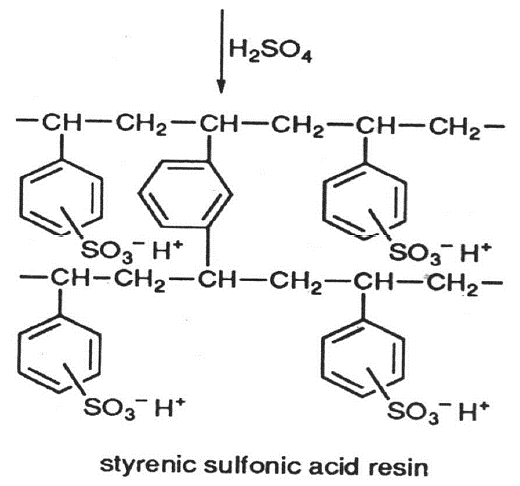
The sulphonic functional group (-SO3H) has an affinity for various metal ions depending on the valency and the ionic size. The higher the valency and the ionic size the higher the affinity. The affinity series for these metal ions is as follows (in descending order): Pu4+ >> La3+ > Ce3+ > Pr3+ > Nd3+ > Sm3+ > Eu3+ > Gd3+ > Tb3+ > Dy3+ > Ho3+ > Er3+ > Tm3+ > Yb3+ > Lu3+ > Y3+ > Sc3+ > Al3+ >> Ba2+ > Pb2+ > Sr2+ > Ca2+ > Ni2+ > Cd2+ > Cu2+ > Co2+ > Zn2+ > Mg2+ > UO22+ >> Tl+ > > Ag+ > Cs+ > Rb+ > K+ > NH4+ > Na+ > H+ > Li+.
Unwanted heavy metal cations present in water e.g. lead, silver, cadmium, etc. will be removed using this cationic exchange resin and substituted for hydrogen ions which react with carbonate and bicarbonate ions thus yielding carbon dioxide. Strong acidic exchange resins work best at optimum pH conditions of between 7 and 13 i.e. basic. The allowable temperature range is usually between 10oC and 100oC for both cationic and anionic exchange resins.
Quaternary amine exchange resin formation:
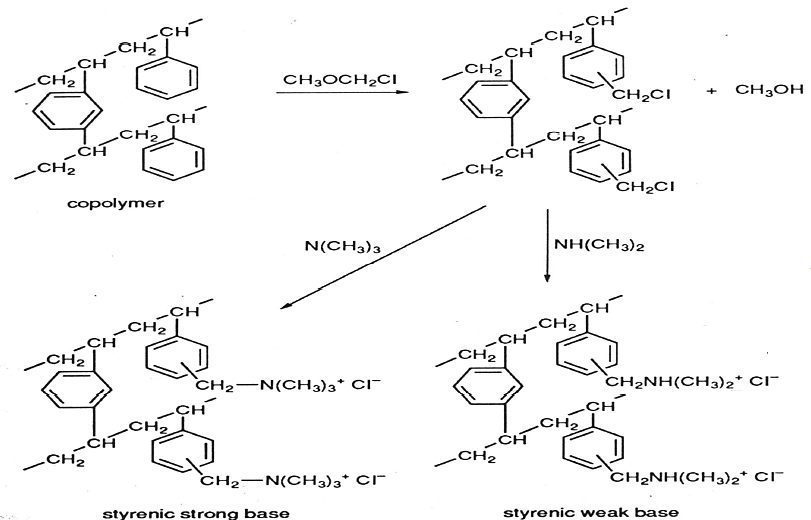
The affinity series of the quaternary amine functional group is: citrate > tartrate > PO43- > AsO43- > ClO4– > SCN– > I– > S2O32- > WO42- > MoO42- > CrO42- > C2O42- > SO42> SO32-> HSO4– >HPO42- > NO3– > Br– > NO2– > CN– > Cl– > HCO3– > H2PO4– > CH3COO– > IO3– > HCOO– > BrO3– > ClO3– > F– > OH–. Industrial pollutants such as arsenate, chromate, cyanide and bromates will be replaced with hydroxide ions thus purifying the water. Strong basic anion exchange resins function best at a pH range of (2–9).
These exchange resins get depleted over time and need regeneration to restore the counter ions. For the sulphonic exchange resin, hydrogen ions (H+) will be restored by running a solution of 100% hydrochloric acid (HCl), whereas sodium hydroxide is used to regenerate hydroxide ions (OH–) in the anion exchange resin.
Ion exchange resins, however, face various challenges such as organic fouling and mechanical stress due to high flow rates. To overcome this challenge, our proposed system will use macro-porous narrow grade resins. The figure below shows the structure of a macro-porous resin.
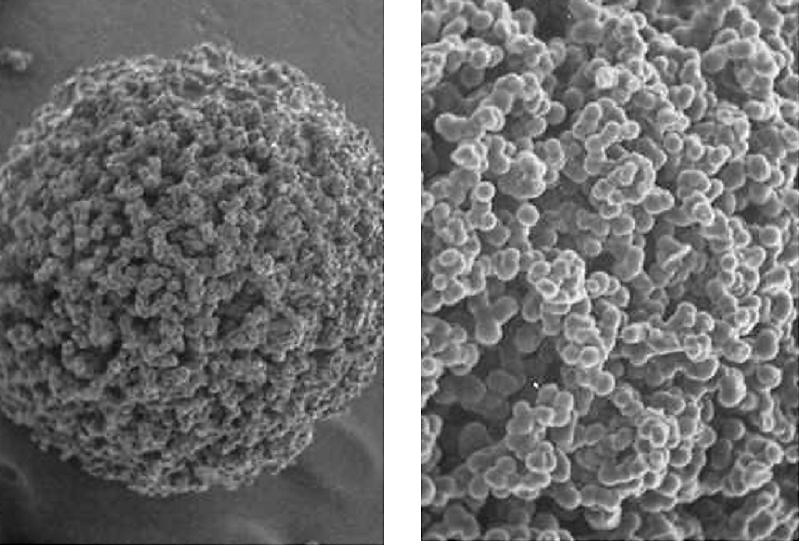
This type of resin will facilitate:
- Higher ion exchange capacity due to a large surface area.
- Better chemical, thermal and mechanical resistance than conventional resins.
- Better drainage conditions. The macro-porous structure will improve the drainage conditions of the narrow grade resin.
- Adsorption of organics and other pollutants due to the macro-porous structure.
The polymer backbone will be made of 8% divinylbenzene for optimal performance and economic viability.
Pumpless Ion-exchange System (PIES)
The ion exchange process can be performed using two techniques: column (continuous flow) or batch technique. Our system is based on the column technique where two columns will be used to carry out the cation and anion exchange processes. Unlike other systems, our system will not use a pump to maintain a continuous flow between the two columns. The continuous flow will be facilitated by:
· Maintaining the head of decationised water at a constant height.
· Locating the inlet of the anion exchange tower below the outlet of the cation exchange tower.
This is illustrated in the figure below:
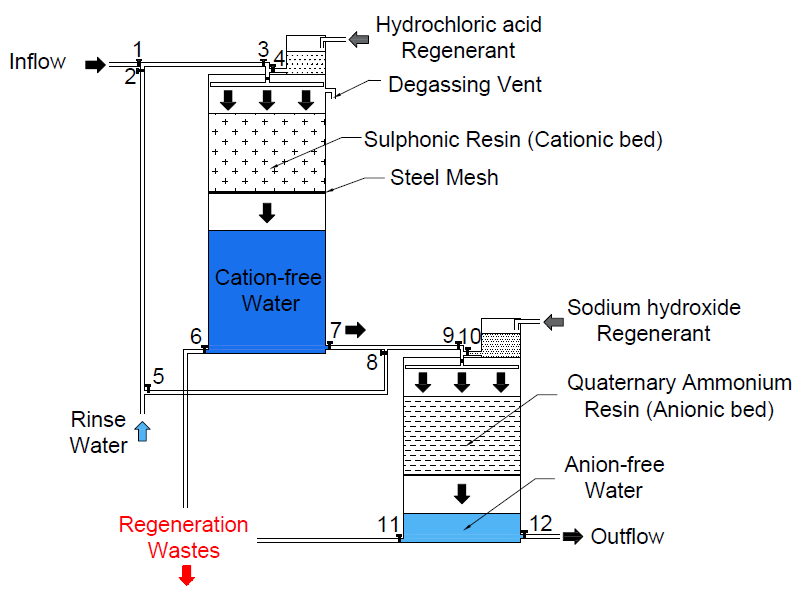
Water is supplied into the towers using a rotary spray nozzle system to ensure even distribution and sufficient contact time. The thickness of the cationic and anionic resin beds is designed to facilitate optimum contact time with the water. The flow rates in the cationic and anionic beds were designed to ensure sufficient contact time and to minimize mechanical stress on the resin beads. The discharge rate of decationised water i.e. at valve 7 will be equal to the flow rate in the cationic bed to maintain an optimum head that will ensure a continuous flow to the anion exchange column. The system is fitted with numerous valves for flow regulation as the piping system is shared by the inflow, rinse water and regenerants. This will prevent mixing of different flows during the process. Valves 6 and 11 are for draining the regenerants and rinse water after regeneration. Valve 5 is used to regulate the flow of pumped rinse water to ensure that both towers are rinsed simultaneously. This is because water tends to travel a shorter head to the anion exchange tower and therefore has to be regulated to ensure equal distribution between the two towers during rinsing. The cation exchange tower has a degassing vent to allow for release of carbon dioxide gas.
The system is ideal for communal water treatment where water from elevated water storage tanks will be treated before being stored in a secondary water storage tank from where it will be supplied to households. The flowchart below shows an overview of the process:
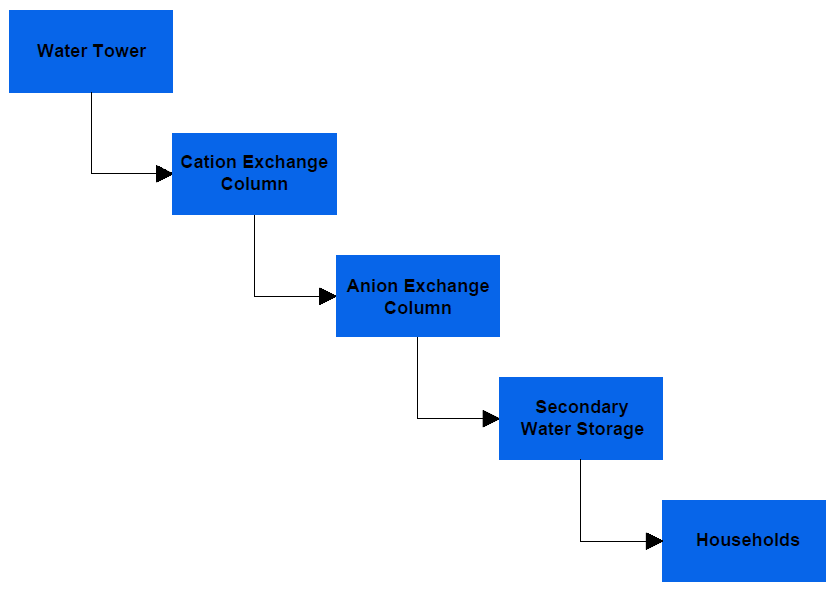
With various adjustments, this system can be integrated into a large water treatment plant. For proper design of an ion-exchange system, water analysis should be carried out to provide information on the amount of cations and anions present in a given volume of water. This will facilitate determination of the following system properties:
- Flow rate
- Vessel size (area)
- Resin volume (bed thickness)
- Amount of regenerant required
- Contact time
- Cycle time
- Regeneration time
Why PIES?
- 99% removal of harmful ions thus making water safe for human consumption.
- Low cost water purification method. According to SAMCO, a fully equipped ion exchange system can cost between $200,000 and $300,000 depending on the size. PIES of a similar size will be cheaper both in terms of initial and operating costs since it is simple, and does not run on a pumping mechanism. According to our calculations, PIES will save approximately $300,000 in pumping costs a year compared to conventional ion exchange units. With the achievement of clean and safe drinking water, the residents of Ulsan won’t have to buy water for drinking and cooking purposes. This will result in savings of up to $10,000,000 a year on the lower side.
- Can be scaled up for large water treatment plant.
- Long-life resin beads.
- The resins used can also act as adsorbents for adsorption of organic pollutants.
- Environmentally sustainable as regenerant wastes will be decontaminated using processes such as metal recovery.
Conclusion
The pumpless ion-exchange system will provide safe drinking water to the people of Ulsan by removing harmful industrial pollutants that initially made the water inconsumable. We believe it is the right solution to the problem as it meets the aspects of desirability, viability, feasibility and sustainability.
We therefore take this opportunity to invite partners, both from the technical and business fields to join hands with us and make PIES a reality!
References
Windsor B., (2012). Resin Types and Production. https://www.soci.org/-/media/Files/Conference-Downloads/2012/IEX-Intro-Water-Sept-2012/Brian_Windsor_resin.ashx?la=en
Windsor B., (2012). Ion Exchange Design – Hand Calculation. https://www.soci.org/-/media/Files/Conference-Downloads/2012/IEX-Advanced-Water-course-Sept-2012/Brian_Windsor_calculation.ashx?la=en
Hubicki, Z., & Kołodyńska, D., (2012). Selective Removal of Heavy Metal Ions from Waters and Waste Waters Using Ion Exchange Methods. In (Ed.), Ion Exchange Technologies. IntechOpen. https://doi.org/10.5772/51040
Michaud, C.F., (2013). Hydrodynamic Design, Part 8: Flow Through Ion Exchange Beds. Water Conditioning & Purification (WC&P) International Magazine. https://wcponline.com/2013/08/06/hydrodynamic-design-part-8-flow-ion-exchange-beds/
SAMCO, (2017). How Much Does an Ion Exchange System Cost?





